Visual Representation of the Experiment
Alka seltzer food coloring experiment – The Alka-Seltzer and food coloring experiment offers a visually striking demonstration of a chemical reaction. The interplay of colors, the rapid release of gas, and the overall dynamism of the process make it an engaging way to observe basic chemical principles in action. The visual changes directly reflect the underlying chemical reactions, providing a clear and accessible link between the macroscopic and microscopic levels.The visual changes observed are dramatic and easily tracked.
Initially, the water in the glass or container is clear, perhaps with a slight tinge of color from the food coloring already added. Upon dropping the Alka-Seltzer tablet, a rapid fizzing action begins, immediately producing carbon dioxide gas in the form of bubbles. These bubbles rise to the surface, creating a visually appealing, effervescent effect. Simultaneously, the food coloring, initially concentrated near the tablet, begins to disperse, creating swirling patterns and gradients of color throughout the water.
The intensity of the color may also appear to change slightly as the concentration of dye alters with the mixing action of the rising bubbles. The entire process is dynamic and relatively quick, creating a visually interesting spectacle.
Image Description: An Alka-Seltzer and Food Coloring Reaction
Imagine a clear glass cylinder, about half-filled with water. A few drops of bright blue food coloring have been added, resulting in a pale blue tint throughout the water. At the bottom center of the glass, a white Alka-Seltzer tablet is partially submerged, releasing a steady stream of tiny, rapidly rising bubbles. These bubbles are visibly carrying and dispersing the blue dye, creating swirling patterns of lighter and darker blue hues.
The water near the tablet is a slightly more intense blue, while the top of the water column is a paler shade. The overall appearance is one of lively, chaotic movement, with the bubbles acting as tiny, buoyant vehicles carrying the color throughout the liquid. The gas bubbles escaping at the surface create a gentle disturbance, showing the release of carbon dioxide.
The entire scene is dynamic and visually engaging, illustrating the chemical reaction clearly.
Visual Changes and Chemical Reactions
The visual changes are directly related to the chemical reaction between the sodium bicarbonate (baking soda) and citric acid present in the Alka-Seltzer tablet. When the tablet dissolves in water, these two compounds react, producing sodium citrate, water, and carbon dioxide gas. The equation for this reaction is:
3NaHCO3(aq) + C 6H 8O 7(aq) → 3CO 2(g) + 3H 2O(l) + Na 3C 6H 5O 7(aq)
The rapid production of carbon dioxide gas is what causes the fizzing and the formation of bubbles. These bubbles, in turn, mix the food coloring, causing the observed color changes and swirling patterns. The dispersal of the color is a passive process driven by the convective currents generated by the rising bubbles, not a direct result of the chemical reaction itself.
The intensity of the color changes reflects the concentration of the dye in different areas of the container, as it is carried by the rising gas bubbles. The visual effect clearly demonstrates the energy released during the exothermic reaction.
Safety Precautions and Considerations
This Alka-Seltzer and food coloring experiment, while visually appealing and educational, involves materials that require careful handling to ensure a safe and successful outcome. Ignoring safety precautions can lead to minor inconveniences like spills or more serious issues, so it’s crucial to prioritize safety throughout the process.Proper safety procedures are essential to minimize risks and promote responsible scientific exploration.
This section Artikels potential hazards and the steps needed to prevent accidents, as well as the appropriate disposal of materials used in the experiment.
Potential Hazards and Mitigation Strategies
The primary potential hazards in this experiment stem from the materials used: Alka-Seltzer tablets, food coloring, and water. Alka-Seltzer tablets, while generally safe, can cause irritation if ingested. Food coloring, though typically non-toxic, can stain clothing and surfaces. Spilled liquids can create a slipping hazard.To mitigate these risks, always conduct the experiment in a well-ventilated area, preferably over a sink or tray to catch any spills.
Wear protective eyewear to shield your eyes from splashes. Ensure adult supervision, especially for younger participants. Avoid direct contact with the Alka-Seltzer solution and immediately wash any spills with soap and water. Use a small amount of food coloring to minimize the risk of staining.
The Alka-Seltzer food coloring experiment demonstrates the principles of chemical reactions through effervescence. A similar, visually captivating experiment uses surface tension to showcase color mixing; check out this milk food coloring experiment for a fascinating comparison. Both experiments offer engaging ways to explore scientific concepts at home, highlighting the diverse reactions of common household items.
Responsible Disposal of Materials
After the experiment, proper disposal of materials is crucial. The used Alka-Seltzer solution, once the reaction has subsided, can be safely rinsed down the drain with plenty of water. Any remaining Alka-Seltzer tablets should be disposed of in the trash. Remember to thoroughly clean any glassware or containers used in the experiment.
Safety Guidelines
- Always wear safety goggles to protect your eyes from splashes.
- Conduct the experiment in a well-ventilated area.
- Perform the experiment over a tray or sink to contain spills.
- Avoid direct contact with the Alka-Seltzer solution.
- Wash hands thoroughly after the experiment.
- Dispose of materials responsibly, rinsing the solution down the drain with plenty of water and discarding tablets in the trash.
- Adult supervision is recommended, especially for children.
- Clean up any spills immediately.
- Use appropriate caution when handling glassware to avoid breakage.
Further Exploration and Extensions

This Alka-Seltzer and food coloring experiment provides a fantastic foundation for exploring a range of scientific concepts. Building upon the observations made, students can delve deeper into chemical reactions, density, and even the principles of diffusion. Adapting the experiment for different age groups and educational levels is straightforward, allowing for a versatile learning experience. Furthermore, the underlying principles have practical applications in various real-world scenarios.The experiment’s inherent simplicity allows for several extensions, making it ideal for a multi-faceted approach to science education.
By altering variables and incorporating additional elements, students can gain a richer understanding of the processes involved. This adaptability makes it suitable for both younger learners focusing on basic observation and older students tackling more complex concepts.
Adapting the Experiment for Different Age Groups
Younger children (elementary school) can focus on the visual aspects, observing the color changes and gas production. They can document their observations through drawings or simple written descriptions. Older children (middle school) can begin to incorporate more quantitative measurements, such as the volume of gas produced or the time it takes for the reaction to complete. High school students can explore the chemical reactions in more detail, researching the specific chemical compounds involved and writing balanced chemical equations.
They might even design their own variations of the experiment to test specific hypotheses. For example, varying the amount of Alka-Seltzer or water could lead to quantitative analysis of reaction rates.
Additional Experiments Building Upon the Alka-Seltzer Experiment, Alka seltzer food coloring experiment
Several related experiments can enhance understanding. For example, comparing the reaction rates of Alka-Seltzer in different liquids (e.g., water, juice, milk) demonstrates the effect of solvent properties on reaction speed. Investigating the effect of temperature on the reaction rate would further solidify the understanding of reaction kinetics. Another interesting extension involves using different concentrations of Alka-Seltzer to observe the relationship between reactant concentration and reaction rate.
The effect of surface area can be explored by comparing the reaction rate of whole tablets versus crushed tablets.
Real-World Applications of the Scientific Principles
The principles demonstrated in this experiment—chemical reactions, gas production, and density—have numerous real-world applications. The production of carbon dioxide gas, as observed in the experiment, is crucial in processes like baking (leavening agents) and in carbonated beverages. Understanding reaction rates is essential in various industrial processes, such as the manufacturing of pharmaceuticals and the development of efficient catalysts. The concept of density, visualized by the layers of colored water, plays a significant role in meteorology (atmospheric layering) and oceanography (ocean stratification).
The principles of diffusion, observed in the gradual mixing of colors, are relevant in various biological processes, such as the transport of oxygen in the blood. For instance, the fermentation process in baking bread relies heavily on the production of carbon dioxide gas, mirroring the gas production observed in the Alka-Seltzer experiment. The rising of bread is a direct consequence of this gas production, showcasing a real-world application of the same chemical principle.
Commonly Asked Questions: Alka Seltzer Food Coloring Experiment
Can I use different types of tablets besides Alka-Seltzer?
While Alka-Seltzer works best due to its specific formulation, you can experiment with other effervescent tablets, but results may vary.
What if the reaction is too slow or too fast?
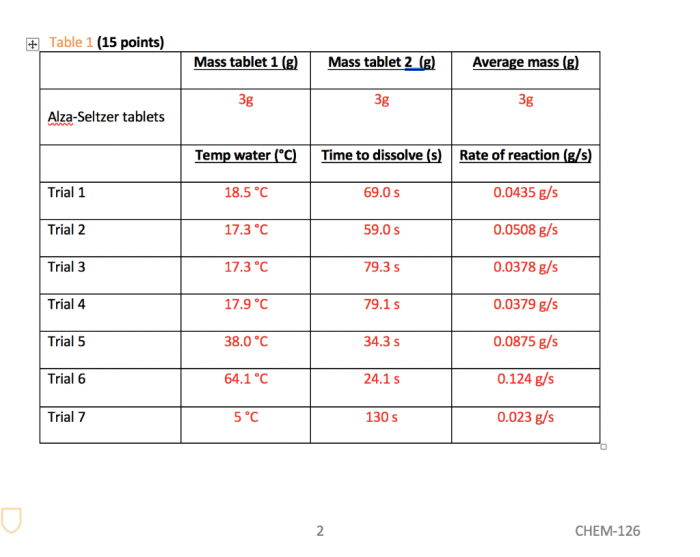
Adjust the water temperature (warmer water speeds it up) or the amount of tablet used to control the reaction rate.
What happens if I use too much food coloring?
Too much food coloring might obscure the visibility of the bubbles and make it harder to observe the reaction clearly. Start with a small amount and add more as needed.
How do I dispose of the materials safely?
Pour the solution down the drain with plenty of water. Rinse all containers thoroughly.
