Introduction to the Baking Soda, Vinegar, and Food Coloring Experiment

Baking soda vinegar food coloring experiment – This experiment demonstrates a classic acid-base reaction, visually engaging and easily performed at home. It’s a fantastic way to introduce young learners to the concepts of chemical reactions and observation of physical changes. The reaction produces a gas, creating a visually exciting outcome that helps solidify understanding.The chemical reaction between baking soda (sodium bicarbonate) and vinegar (acetic acid) is exothermic, meaning it releases energy in the form of heat, though the temperature change is usually not significant enough to be easily felt.
The reaction produces carbon dioxide gas, water, and sodium acetate.
Yo, so you’re doing that baking soda and vinegar volcano thing with food coloring, right? It’s totally rad, but sometimes you need a specific shade, like a chill light brown. If you’re tryna get that perfect color, check out this tutorial on how to make light brown food coloring to make your science experiment even more epic.
Then, boom, you’ll have the perfect brown eruption for your next baking soda volcano project!
Materials Required for the Experiment
This experiment requires readily available household materials. Gathering the necessary items beforehand ensures a smooth and efficient experimental process. Having everything organized will minimize interruptions and maximize the learning experience.
- Baking soda (sodium bicarbonate)
- White vinegar (acetic acid)
- Food coloring (various colors)
- Clear container (e.g., a glass or plastic bottle)
- Measuring spoons and cups (for precise measurements, though exact amounts are flexible)
The Chemical Reaction Between Baking Soda and Vinegar
The core of this experiment lies in the reaction between baking soda and vinegar. This is an acid-base neutralization reaction. Baking soda, a base, reacts with the acid in vinegar (acetic acid) to produce carbon dioxide gas, water, and sodium acetate. The carbon dioxide gas is what causes the fizzing and bubbling observed during the experiment.
NaHCO3 (baking soda) + CH 3COOH (vinegar) → CO 2 (carbon dioxide) + H 2O (water) + CH 3COONa (sodium acetate)
This equation shows that one molecule of sodium bicarbonate reacts with one molecule of acetic acid to produce one molecule each of carbon dioxide, water, and sodium acetate. The released carbon dioxide gas is less dense than air, causing it to bubble up and escape the solution.
Expected Outcome of the Experiment
When baking soda is added to vinegar, a rapid chemical reaction occurs, resulting in the immediate production of carbon dioxide gas. The addition of food coloring enhances the visual impact of the experiment, creating a colorful, bubbling eruption. The intensity of the reaction, as measured by the amount of fizzing and the height of the foam produced, will depend on the relative amounts of baking soda and vinegar used.
For instance, using a larger quantity of baking soda will lead to a more vigorous reaction, creating a larger volume of foam. Conversely, using less baking soda will result in a less dramatic, but still observable, reaction. The experiment consistently demonstrates the principles of acid-base chemistry and gas production.
Procedure and Methodology: Baking Soda Vinegar Food Coloring Experiment
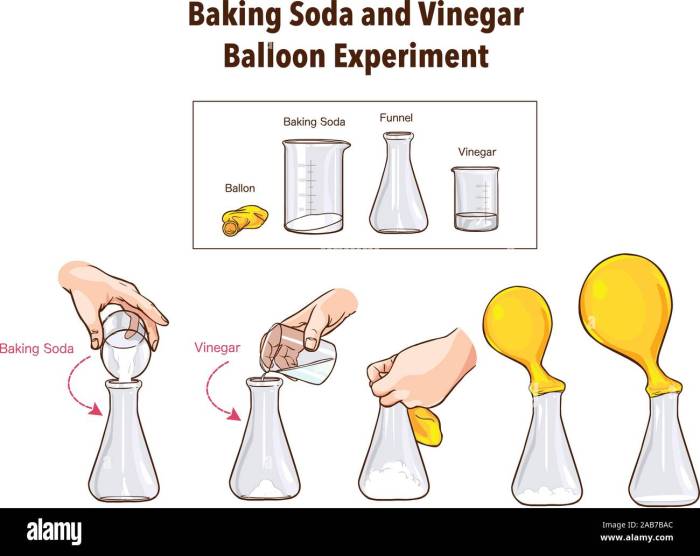
This section details the step-by-step procedure for conducting the baking soda, vinegar, and food coloring experiment, including materials, expected observations, and safety precautions. A clear understanding of the process is crucial for successful and safe experimentation. The experiment is designed to be simple and visually engaging, demonstrating a classic chemical reaction.
The experiment involves combining baking soda (a base) and vinegar (an acid) to create a chemical reaction that produces carbon dioxide gas. The food coloring helps visualize the reaction and the resulting foam.
Experimental Steps, Materials, Observations, and Safety
The following table Artikels the procedure, materials used at each step, expected observations, and relevant safety precautions.
| Step | Materials | Expected Observations | Safety Precautions |
|---|---|---|---|
| 1. Preparation | Bottle, Baking soda, Vinegar, Food coloring, Measuring spoons, Tray or container | Materials are gathered and prepared for the experiment. | Ensure the work surface is clear and stable. Adult supervision is recommended, especially with young children. |
| 2. Adding Baking Soda | Baking soda, Measuring spoon | A specific amount of baking soda (e.g., 1-2 tablespoons) is added to the bottle. | Avoid inhaling baking soda dust. |
| 3. Adding Food Coloring | Food coloring, Dropper or spoon | A few drops of food coloring are added to the baking soda in the bottle. This will color the resulting foam. | Handle food coloring carefully to avoid spills and stains. |
| 4. Adding Vinegar | Vinegar, Measuring cup or beaker | Slowly add vinegar to the bottle containing the baking soda and food coloring. A fizzing reaction will occur, producing carbon dioxide gas and a foamy eruption. The foam will be colored according to the food coloring used. | Add vinegar slowly to control the reaction’s intensity. Avoid direct contact with the mixture. |
| 5. Observation and Cleanup | None | Observe the reaction carefully. The height and color of the foam can be noted. | Allow the reaction to complete before cleaning up. Dispose of the mixture appropriately. Wash hands thoroughly after handling the materials. |
Visual Representation of the Experimental Setup
The visual representation would show a clear bottle or container placed on a tray or container. Inside the bottle, there is a layer of baking soda at the bottom, followed by a few drops of food coloring, creating a visible colored layer on top of the baking soda. The vinegar is shown being carefully poured into the bottle from a separate measuring cup or beaker.
The illustration would depict the resulting foamy eruption, with the colored foam overflowing from the bottle and onto the tray, clearly showing the gas production and the color of the foam.
Variations of the Experiment
This classic baking soda and vinegar reaction offers a surprisingly versatile platform for exploration. By altering the ingredients and their quantities, we can observe a range of fascinating visual and physical changes, deepening our understanding of the chemical process at play. These variations allow for adaptable experimentation, suitable for different age groups and learning levels.The fundamental reaction remains consistent: acetic acid in vinegar reacts with sodium bicarbonate (baking soda) to produce carbon dioxide gas, water, and sodium acetate.
However, manipulating the variables alters the rate and intensity of gas production, resulting in observable differences in the eruption’s height, speed, and visual appeal.
Different Types of Vinegar
Using different types of vinegar, such as apple cider vinegar, white distilled vinegar, or balsamic vinegar, will affect the reaction’s vigor. The concentration of acetic acid varies between vinegar types. White distilled vinegar typically has a higher concentration than apple cider vinegar, leading to a more vigorous reaction. Balsamic vinegar, with its added sugars and other components, might produce a slightly slower and potentially less dramatic reaction due to the presence of other substances interfering with the main reaction.
We can expect to see differences in the speed and height of the eruption, with white distilled vinegar potentially producing the most forceful eruption and balsamic vinegar the least. For instance, a comparison experiment using 100ml of each vinegar type with the same amount of baking soda would demonstrate this clearly. The white vinegar would likely produce a taller, faster eruption, while the balsamic vinegar might produce a slower, less intense fizz.
Varying Amounts of Baking Soda and Vinegar, Baking soda vinegar food coloring experiment
Altering the ratio of baking soda to vinegar directly impacts the amount of carbon dioxide gas produced. A larger quantity of baking soda, with a fixed amount of vinegar, will lead to a more substantial eruption, provided there’s enough vinegar to react with all the baking soda. Conversely, increasing the amount of vinegar while keeping the baking soda constant might result in a less intense but potentially longer-lasting reaction, as the excess vinegar continues to react with the available baking soda.
Imagine using a fixed amount of vinegar (e.g., 100ml) and varying the baking soda from 1 teaspoon to 3 teaspoons. The eruption’s height and intensity will increase proportionally with the baking soda until all the vinegar is consumed.
Adding Different Food Colorings
Adding food coloring allows for a visually captivating element to the experiment. Different colors will create varied and aesthetically pleasing eruptions. The color intensity will depend on the concentration of the food coloring used. The reaction itself is unaffected by the addition of food coloring; it merely alters the visual aspect. Using multiple colors simultaneously can lead to interesting swirling patterns within the erupting foam.
For example, layering different colored solutions in a container before adding the baking soda could create a visually stunning, layered effect as the reaction progresses. The resulting eruption would showcase the mixing of colors in a dynamic way.
Extending the Experiment
The baking soda and vinegar reaction provides a fantastic springboard for numerous scientific explorations. Building upon the foundational understanding of this classic experiment allows students to delve deeper into concepts like chemical reactions, gas production, and even engineering principles. By extending the experiment, we can foster a deeper appreciation for the scientific method and encourage further inquiry.The core principles observed in the initial baking soda and vinegar reaction can be expanded to investigate a range of related phenomena.
This allows for a more comprehensive understanding of the chemical process and opens doors to more complex experiments. Furthermore, integrating this experiment into a broader science curriculum allows for the application of learned concepts in different contexts.
Exploring Reaction Rates
The speed at which the baking soda and vinegar reaction occurs can be systematically altered. Factors such as temperature, concentration of reactants, and surface area of the baking soda can be manipulated to observe their effect on the rate of gas production. For example, comparing the reaction rate of baking soda and vinegar at room temperature versus in an ice bath will demonstrate the temperature’s influence on reaction kinetics.
Similarly, using finely powdered baking soda versus larger crystals will illustrate the impact of surface area. This investigation aligns with the concept of reaction kinetics and allows students to visualize how environmental factors can influence chemical processes.
Building a Simple Volcano Model
The baking soda and vinegar reaction can be visually enhanced and used to create a model volcano. A simple volcano can be constructed using clay or papier-mâché, with a central cavity to contain the baking soda and vinegar. Adding food coloring to the vinegar intensifies the visual effect, simulating a volcanic eruption. This engaging activity combines science with art and allows for creative expression while reinforcing the understanding of the chemical reaction.
The eruption itself visually demonstrates the rapid release of carbon dioxide gas, mirroring the pressure build-up within a real volcano.
Investigating Different Acids
While vinegar (acetic acid) is commonly used, the experiment can be extended to explore other acids. Lemon juice (citric acid) or even diluted hydrochloric acid (with appropriate safety precautions) can be substituted for vinegar to compare the reaction rate and gas production with different acids. This comparison highlights the role of acid strength in the reaction, expanding the students’ understanding beyond a single acid-base reaction.
This exploration reinforces the concept that different acids possess varying strengths and react differently with bases.
Measuring Gas Production
Quantifying the amount of carbon dioxide gas produced during the reaction adds a quantitative element to the experiment. This can be accomplished using a simple apparatus, such as an inverted graduated cylinder filled with water, to collect the gas. By measuring the volume of displaced water, students can directly measure the amount of carbon dioxide produced under different experimental conditions.
This allows for a more precise analysis of the reaction and facilitates the development of data analysis skills. The collected data can then be used to create graphs and draw conclusions about the effect of different variables on the reaction.
FAQ Resource
Can I use different types of food coloring?
Absolutely! Experiment with different colors and see how they affect the visual outcome. Liquid food coloring works best.
What happens if I use too much baking soda?
A bigger, faster reaction, potentially overflowing your container! Start small and adjust.
What if I don’t have white vinegar?
Apple cider vinegar or other types of vinegar will still work, but the results might vary slightly in color.
Is this experiment safe for young children?
Adult supervision is always recommended, especially with young children, to prevent spills and ensure safe handling of materials.
